Structure of the Chaperonin Protein
Structure of the Chaperonin Protein
Submitted by Oksana Sergeeva and Jonathan King in the King Lab at MIT
MIT Department of Biology
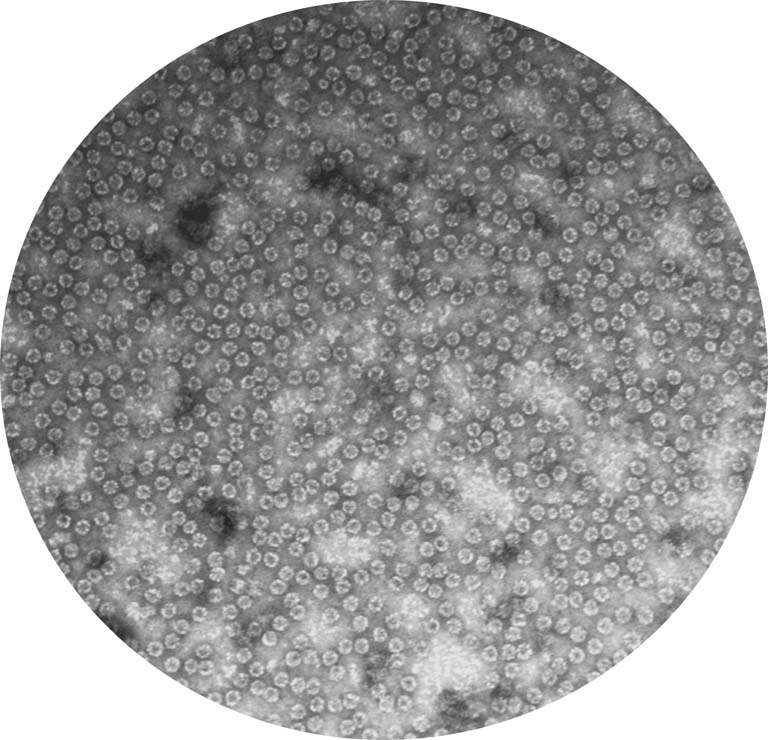
This is the structure of a protein called a chaperonin that folds other proteins by encapsulating them into an inner cavity and then later spitting them out. This particular chaperonin is found in all of our (human) cells and is necessary to keep our cells alive. We isolated this chaperonin (TRiC: TCP-1 Ring Complex) from HeLa cells which had never been done before, so we took this image to check that the structure of this protein was similar to other structure of TRiC isolated from other sources. We hoped to see that it was a double ring barrel, like we see here. Knowing the structure of a protein we are isolating and studying helps us learn more about how it exists and functions in the cell.